OncoK9®—一种基于血液学的犬多种癌症早期检测的“液体活检”检测
Clinical Validation of OncoK9®, a Blood-Based Multi-Cancer Early Detection “Liquid Biopsy” Test for Dogs
翻译:乔宇静 华中农业大学
癌症是导致犬死亡的主要原因1,但目前还没有建立起早期检测的筛选模式。因此,许多病犬可能在晚期才被诊断出来,但此时临床症状已经出现,能够进行长期控制的可能性低,通常预后不良。液体活组织检查方法可以检查血液中2游离DNA(cfDNA) 片段中癌源性基因组改变,这种方法在人类医学3中被用于早期癌症检测,现在也可用于兽医临床4 。犬类癌症检测(CANDiD) 研究是一项国际多中心临床研究,旨在验证OncoK9的性能,OncoK9是一种新型的多癌早检(MCED)“液体活检”检测方法,采用血液来源DNA的下一代测序(NGS)技术,专门用于犬类癌症的无创检测和定性筛查。本白皮书概述了CANDiD研究的主要发现a。
INTRODUCTION
Cancer is the leading cause of death in dogs1 , yet there are no established screening paradigms for early detection. As a result, many patients may be diagnosed at an advanced stage, when clinical signs have developed, the ability to provide long-term control is low, and prognosis is poor.
Liquid biopsy methods that interrogate cancer-derived genomic alterations in cell-free DNA (cfDNA) fragments in blood2 are being adopted for early cancer detection in human medicine3 and are now clinically available for use in veterinary medicine4 . The CANcer Detection in Dogs (CANDiD) study is an international, multi-center clinical study to validate the performance of OncoK9, a novel, multi-cancer early detection (MCED) “liquid biopsy” test using next-generation sequencing (NGS) of blood-derived DNA specifically developed for the noninvasive detection and characterization of cancer in dogs. Key findings from the CANDiD study are summarized in this white paper.a
OncoK9是一种多种癌症的早期检测方法,利用下一代基因测序技术,对从犬类全血样本中分离出的DNA中与癌症有关的基因组改变进行检测和定性。OncoK9旨在用于患癌风险较高的犬。建议所有品种的犬从8岁开始进行年度筛查测试,并且对于易患癌症的品种,可能需 要从更早年龄段开始筛查。它还被推荐作为一种辅助诊断方法, 用于那些根据临床症状或其它临床发现疑似患有癌症的犬。与任何实验室检测一样,OncoK9的结果应该由兽医根据每个患病动物的病史和临床表现来解释。该检查项目只能通过处方开具。
INTENDED USE
OncoK9 is a multi-cancer early detection test for the detection and characterization of cancer-associated genomic alterations in DNA isolated from canine whole blood samples, using next-generation sequencing (NGS) technology. OncoK9 is intended for use in dogs who are at higher risk of cancer. It is recommended as an annual screening test for all dogs starting at 8 years of age and potentially at younger ages for dogs belonging to breeds that are predisposed to cancer. It is also recommended as an aid-in-diagnosis for dogs in which cancer is suspected based on clinical signs or other clinical findings. As with any laboratory test, OncoK9 results should be interpreted by a veterinarian in the context of each patient’s medical history and clinical presentation. This test is available by prescription only.
在进入临床验证之前,试验分析效果通过人为样本进行评估,这些样本是将来自特征明确的犬类癌细胞系的DNA与从单个无癌受试犬的白血球中提取的DNA按规定的基因组进行等效比例混合而成。重复性的评估是通过分析由相同操作者在相同条件下处理的多个运行内重复样本之间的结果一致性。再现性的评估是通过分析在多个运行、操作者、天数和试剂批次条件下处理的样本之间的一致性。重复性和再现性均大于95%。
OncoK9在CANDiD研究中的临床验证包含了在2019年11月至2021年8月期间,从美国、加拿大、巴西、荷兰、法国和香港的41个临床站点预先收集的共1100份客户自养犬(有癌症或 没有癌症)的全血样本b 。采集地点包括私人兽医专科诊所、大学/研究院的兽医院和普通诊所。
纳入测试验证的1100名受试犬符合研究注册和实验室质量控制标准,并获得了测试结果。受试犬被分成独立的训练组 (n=224)和测试组(n=876)。首先使用训练组数据对生物学算法进行优化,之后将研究流程应用于 测试组以确定临床效果。在检测结果发布之前,所有数据审查员对患病动物的癌症状态和癌症类型均不知情。
测试组由352只诊断为癌症的犬(明确诊断为恶性肿瘤的受试犬)和524只假定无癌症的犬(受试犬由于没有癌症病史,并且根据研究登记时兽医给出的全面病史和身体检查,没有患癌的嫌疑,因此假定为无癌症)组成,测试性能由此得出。
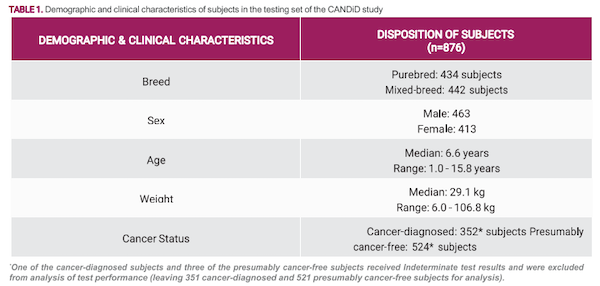
测试组中的癌症确诊对象代表了癌症的全部阶段,其中205例(58%)为局部/区域性病例 c,136例(39%)为扩散/转移性病例d,11例(3%)为疾病程度不确定的病例e 。表1总结了测试集中所有受试犬的群体特征和临床特 征。
CLINICAL VALIDATION OF ONCOK9
Prior to clinical validation, analytical performance of the test was assessed using contrived samples, generated by mixing DNA from well-characterized canine cancer cell lines into DNA extracted from the white blood cells of a single cancer-free canine subject at defined genomic equivalent ratios. Repeatability was assessed by analyzing agreement among multiple within-run replicates, processed by the same operators under the same conditions.Reproducibility was assessed by analyzing agreement among replicates across multiple runs, operators, days, and reagent lots. The repeatability and reproducibility were each >95%.
Clinical validation of OncoK9 in the CANDiD study included a total of 1,100 prospectively collected whole blood samples from client-owned dogs, with and without cancer, at 41 clinical sites across the US, Canada, Brazil, the Netherlands, France, and Hong Kong between November 2019 and August 2021.b Collection sites included private veterinary specialty practices, university/academic veterinary hospitals, and general practices.
The 1,100 subjects included in the validation of the test met study enrollment and laboratory QC criteria and received a test result. Subjects were separated into independent training (n=224) and testing (n=876) sets. Bioinformatics algorithms were optimized using the training set, and the locked-down pipeline was subsequently applied to the testing set to establish the test’s clinical performance characteristics. All datareviewers were blinded to the cancer status and type of cancer in patients until after test results were issued.
The testing set, from which test performance was derived, comprised 352 cancer-diagnosed dogs (subjects witha definitive diagnosis of a malignant tumor) and 524 presumably cancer-free dogs (subjects presumed to be cancer-free due to no history of cancer and no suspicion of cancer based on a thorough history and physical exam by the treating veterinarian at the time of study enrollment).
The cancer-diagnosed subjects in the testing set represented the full spectrum of cancer stages, with 205 (58%) localized/regionalc cases, 136 (39%) disseminated/ metastaticd cases, and 11 (3%) cases where the extentof disease was indeterminatee . Table 1 summarizes the demographic and clinical characteristics of all subjects in the testing set.
淋巴瘤、血管肉瘤和骨肉瘤是高度侵略性的犬类癌症。在测试组中,共有137名受试犬的单一原发癌症属于这三种癌症类型之一(94例淋巴瘤f,31例骨肉瘤,12例血管肉瘤g )。在这137名受试犬中,有117名受试犬 的液体活检检测结果显示检测到癌症信号(阳性),因此这三种侵袭性癌症类型的总体检出率为85.4% (95%CI: 78.4-90.9%)。(图 1)
ONCOK9 PERFORMANCE IN THREE OF THE MOST AGGRESSIVE CANINE CANCERS
Lymphoma, hemangiosarcoma, and osteosarcoma are highly aggressive canine cancers. In the testing set, there were 137 total subjects with a single primary cancer belonging to one of these three cancer types (94 lymphoma casesf , 31 osteosarcoma cases, 12 hemangiosarcomag cases). The liquid biopsy test returned a Cancer Signal Detected (positive) result in 117 of these 137 subjects, resulting in an overall detection rate of 85.4% (95% CI: 78.4 – 90.9%) across these three aggressive cancer types. (Figure 1)
特定类型的癌症在犬身上更常见,并占该物种癌症死亡率的大部分,尤其是这八种:淋巴瘤f 、血管肉瘤g 、骨肉瘤、软组 织肉瘤、肥大细胞瘤、乳腺癌、 肛门囊腺癌和恶性黑色素瘤5。在测试集中,有236名受试犬的单一原发性癌症属于这八种癌症类型之一,其检测结果为阳性或阴性。在这236名受试犬中,有146名受试犬的液体活检检测结果显示检测到癌症信号(阳性) ,因此这八种常见癌症类型的总体检出率为61.9% (95% CI: 55.3- 68.1%)。(图1)
ONCOK9 PERFORMANCE IN EIGHT OF THE MOST COMMON CANINE CANCERS
Certain types of cancer are more common in dogs and account for the majority of cancer mortality in the species, notably these eight: lymphomaf , hemangiosarcomag , osteosarcoma, soft tissue sarcoma, mast cell tumor, mammary gland carcinoma, anal sac adenocarcinoma, and malignant melanoma5 . In the testing set, there were 236 subjects with a single primary cancer belonging to one of these eight cancer types that received a positive or negative result. The liquid biopsy test returned a Cancer Signal Detected (positive) result in 146 of these 236 subjects, resulting in an overall detection rate of 61.9% (95% CI: 55.3 – 68.1%) across these eight common cancer types. (Figure 1)
在测试组中,有351名确诊癌症的受试犬的结果为阳性或阴性。在这些受试犬中,有192只受试犬的检测结果显示检测到癌症信号(阳性),总体灵敏度( 检出率)为54.7% (95%CI: 49.3- 60.0%)h。OncoK9从433名训练集和测试集中确诊癌症的受试犬检测到30种不同类型癌症的癌症信号。

在测试组中,有521名假定无癌症的受试犬的结果为阳性或阴性。在这些受试犬中,有511 只犬的测试结果是未检测到癌症信号(阴性),有10只犬的测试结果是检测到癌症信号(阳性)(“假定假阳性”,pFP)。两名 pFP受试犬在接受了由癌症信号检测结果引发的确认性癌症评估后被诊断为癌症,并被排除在测试性能分析之外;这两只受试犬 分别在采集血样6个月和7个月后被诊断为癌症。
剩下的519名受试犬被用于计算特异性。该测试的总体特异性为98.5% (511/519; 95% CI: 97.0-99.3%),对应的假阳性率 (FPR)为1.5% (95% CI:0.7-3.0%) i 。(图1)如果任何剩余pFP病例诊断出癌症,这些受试犬也将被排除在测试特异性分析之外, 因为癌症诊断将有效地将这些受试犬从假定的无癌症队列中排除,进一步提高测试的特异性。

ONCOK9 PERFORMANCE FOR MULTI-CANCER DETECTION
There were 351 all-comers cancer-diagnosed subjects in the testing set that received a positive or a negative result. In this cohort, the test returned a Cancer Signal Detected (positive) result for 192 subjects, for an overall sensitivity (detection rate) of 54.7% (95% CI: 49.3-60.0%).h OncoK9 detected cancer signal in 30 distinct cancer types represented by 433 cancer-diagnosed subjects evaluated across the training and testing sets.
There were 521 presumably cancer-free subjects in the testing set that received a positive or a negative result. In these subjects, the test returned a Cancer Signal Not Detected (negative) result for 511 dogs, and Cancer Signal Detected (positive) result for 10 dogs (“putative false positives”, pFP). Two pFP subjects were diagnosed with cancer after undergoing a confirmatory cancer evaluation triggered by a Cancer Signal Detected result, and were excluded from analysis of test performance; these subjects were diagnosed with cancer 6 and 7 months following collection of the blood samples, respectively.
The remaining 519 subjects were used for the calculation of specificity. The overall specificity of the test was 98.5% (511/519; 95% CI: 97.0 – 99.3%), corresponding to a false positive rate (FPR) of 1.5% (95% CI: 0.7 – 3.0%)i . (Figure 1) If adjudication of any of the remaining pFP cases were to yield a cancer diagnosis, those subjects will also be excluded from analysis of test specificity, as a cancer diagnosis would effectively eliminate those subjects from the presumably cancer-free cohort, further increasing the specificity of the test.
按照目前的敏感性和特异性,并假设“筛查”和“辅助诊断”预期使用群体的癌症患病率分别为8-10%和30-50%,可以估计 OncoK9的阳性预测值(PPV)。如表2所示,当出现阳性(检测到癌症信号)结果时,PPV预计在 76%和97%之间(取决于个体的 癌症先验概率),这意味着绝大多数收到OncoK9阳性结果的患宠将被证明为临床上的癌症阳 性。在OncoK9结果为阴性(未 检测到癌症信号)的情况下,阴性预测值(NPV)预计在68%至 96%之间(也取决于个体的癌症先验概率)。这强调了如果液体活检的结果为阴性,但癌症仍然在鉴别诊断名单上高居前列,那么进一步评估则十分重要。
POSITIVE AND NEGATIVE PREDICTIVE VALUES
At the current sensitivity and specificity, and assuming the prevalence of cancer in the “screening” and “aid-indiagnosis” intended use populations is 8-10% and 30- 50% respectively, the positive predictive value (PPV) of OncoK9 can be estimated. As shown in Table 2, when a positive (Cancer Signal Detected) result is issued, the PPV is expected to range from 76 and 97% (depending on the prior probability of cancer in that individual), meaning that a large majority of patients who receive a positive OncoK9 result will prove to be clinically positive for cancer. In the context of a negative (Cancer Signal Not Detected) OncoK9 result, the negative predictive value (NPV) is expected to range from 68 to 96% (also depending on the prior probability of cancer in that individual). This underscores the importance of further evaluation if the liquid biopsy test result is negative but cancer remains high on the list of differential diagnoses.
在收到癌症信号检测结果的受试犬中,在解除盲法之前使用专有算法对血液恶性肿瘤特有的基因组特征进行了测序数据审查。在测试集中,共有96名诊断为血液恶性肿瘤的受试犬得到了癌症信号检测结果。在这96名受试犬中,40%(n=38)被分配到“ 血液恶性肿瘤”的癌症信号起源 (CSO)预测,而其余58只患犬没有得到CSO预测;3名被诊断为非血液恶性肿瘤的受试犬被额外分配到这个CSO预测,得出血液恶性肿瘤的总体CSO预测准确性为92.7% (38/41)。
CANCER SIGNAL ORIGIN’ PREDICTION
In subjects who received a Cancer Signal Detected result, and prior to unblinding, the sequencing data were reviewed using proprietary algorithms for genomic features specific to hematological malignancies. A total of 96 subjects with a diagnosis of hematological malignancy in the testing set received a Cancer Signal Detected result. Of these 96 subjects, 40% (n=38) were assigned a Cancer Signal Origin (CSO) prediction of “hematological malignancy”, while the remaining 58 did not receive a CSO prediction; three subjects who had been diagnosed with non-hematological malignancies were additionally assigned this CSO prediction, for an overall CSO prediction accuracy of 92.7% (38/41) for hematological malignancy.
对于从测试集中得到阳性或阴性结果的癌症确诊受试犬来看,测试的表现与以下任何预分析参数没有显著关联:从收集到处理样本所用的时间(1-7天之间)、溶血程度和脂血程度。样本的采集不受任何时间限制,也不受犬最后一次喂食时间的限制。
EVALUATION OF PRE-ANALYTICAL FACTORS
The performance of the test did not have a significant association with any of the following pre-analytical parameters across the cancer-diagnosed subjects that received a positive or negative result in the testing set: time from collection to processing (between 1-7 days), extent of hemolysis, and extent of lipemia. Samples were collected without any restrictions related to the time of day or the time of the dog’s last feeding.
OncoK9是一种新型的、基于血液的多癌早检(MCED)测试,用于检测和确定犬的癌症特征类型。在训练组和测试组的所有癌症确诊对象中,OncoK9在30种不同的癌症类型中的每个癌症类型中至少检测到一个受试犬的癌症信号,显示了强大的多癌检测能力,建议将其作为一种无创方法应用于多种犬类癌症检测。兽医现在可以获得一种基于下一代基因测序的癌症检测,其性能可与人类医学中最先进的液体活检检测相媲美。
CONCLUSIONS
OncoK9 is a novel, blood-based multi-cancer early detection (MCED) test for the detection and characterization of cancer in dogs. Across all cancer-diagnosed subjects in the training and testing sets, OncoK9 detected cancer signal in at least one subject per cancer type across 30 distinct cancer types, demonstrating robust multi-cancer detection capabilities and suggesting utility as a noninvasive method for detecting a wide spectrum of canine cancers. Veterinarians now have access to a next-generation sequencing-based cancer test with performance comparable to state-of-the-art liquid biopsy tests in human medicine.3
信息来源:新技术进展
1.Fleming JM, Creevy KE, Promislow DEL. Mortality in North American Dogs from 1984 to 2004: An Investigation into Age‐, Size‐, and Breed‐ Related Causes of Death. J Vet Intern Med. 2011;25(2):187–98. doi: 10.1111/j.1939-1676.2011.0695.x
2.Chibuk J, Flory A, Kruglyak KM, Leibman N, Nahama A, Dharajiya N, et al. Horizons in Veterinary Precision Oncology: Fundamentals of Cancer Genomics and Applications of Liquid Biopsy for the Detection, Characterization, and Management of Cancer in Dogs. Frontiers Vet Sci. 2021;8: 664718. doi:10.3389/ fvets.2021.664718
3.Klein EA, Richards D, Cohn A, Tummala M, Lapham R, Cosgrove D, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncol. 2021. doi:10.1016/j.annonc.2021.05.806
4.Kruglyak KM, Chibuk J, McLennan L, Nakashe P, Hernandez GE, Motalli- Pepio R, et al. Blood-Based Liquid Biopsy for Comprehensive Cancer Genomic Profiling Using Next-Generation Sequencing: An Emerging Paradigm for Non-invasive Cancer Detection and Management in Dogs. Frontiers Vet Sci. 2021;8: 704835. doi:10.3389/ fvets.2021.704835
5.Biller B, Berg J, Garrett L, Ruslander D, Wearing R, Abbott B, et al. 2016 AAHA Oncology Guidelines for Dogs and Cats. J Am Anim Hosp Assoc. 2016;52: 181–204. doi:10.5326/jaaha-ms-6570
a CANDiD研究的全部结果已提交出版,目前仍在接受同行评议。
b 所有受试犬都是在获得机构动物福利伦理委员会 (IACUC)或特定地点的伦理学批准的方案下,根据每个地点的要求入选的。所有受试犬都是客户拥有的,并获得了所有所有者的书面知情同意。
c 在实体瘤中,“局部/区域”是指局限于原发器官或附近的淋巴结、组织或器官的癌症;在淋巴瘤中,该类别是指局限于单个淋巴结(I期)或位于膈肌一侧的多个淋巴结(II期)的癌症。
d 在实体瘤中,“扩散/转移”是指癌症扩散到远离原发肿瘤的身体区域;在淋巴瘤中,这类癌症是指涉及膈肌两侧的两个或多个淋巴结和/或一个或多个结外部位的癌症(第III、IV和V阶段)。
e 不确定是指尽管有完整的癌症分期诊断,但没有足够的证据来确定疾病的程度。
f 包括B细胞和T细胞,以及未分类的(非表型)淋巴瘤;不包括惰性淋巴瘤。
g 包括皮肤的、肌肉内的、腹部内脏的和心脏的血管肉瘤。
h 敏感度:确诊癌症的犬得到检测出癌症信号(阳 性)结果的百分比;也被称为“检出率”。
i 特异性:没有癌症的犬得到未检测到癌症信号( 阴性)结果的百分比。假阳性率等于1减去特异性。